The Liao Lab
The mission of our lab is to make a positive impact in science, in our team members and for patients everywhere. Our research program focuses on understanding the developmental biology and genetic underpinnings of craniofacial development. We are particularly interested in translating fundamental discoveries to clinical impact. Current projects include investigations of transcription factors important in regulating embryonic epithelial biology and neural crest differentiation, and the clinical malformations associated with this biology. We utilize the state-of-art approaches of genomics and cell biology in zebrafish, mouse and human iPSC models to mechanistically analyze these topics. Our lab is highly collaborative and provides a nurturing and fun environment ideal for graduate students interested in learning science, learning the tools to do science and mentorship toward an impactful career using science.
Read the Innovation Story
& View Video

CLINICAL TRANSLATION
-
Cleft and orofacial cleft outcomes
-
Craniosynostosis
-
Beckwith-Weidemann Spectrum
-
Molecular diagnosis and deep phenotyping
-
Fetal diagnosis and congenital personal medicine
-
Drug discovery
-
Gene editing

FUNDAMENTAL DISCOVERY
-
Zebrafish models of cleft lip and palate malformation
-
Cranial neural crest biology
-
Transcriptional regulation of craniofacial development
-
Epithelial mesenchymal transition
-
RNA splicing during embryogenesis
-
High throughput functional analysis of candidate genes implicated in facial morphogenesis
-
Wnt signaling in craniofacial development
Formation of the vertebrate facial structures requires coordination of complex molecular and morphogenetic cues. The genes regulating facial development are well conserved across vertebrate species, where minor molecular variations contribute to dramatic alterations in form.
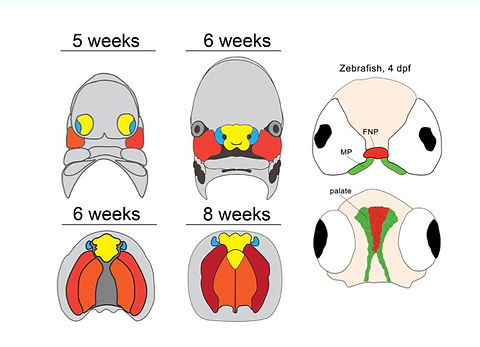
We take advantage of the versatility of forward and reverse genetics in zebrafish as a model to assay function of human cleft candidate genes and demonstrated that the zebrafish palate (ethmoid plate) is morphogenetically homologous to the mammalian primary palate (Dougherty, Development, 2013). We also showed that convergence and extension mechanisms operate in palate morphogenesis, and we are dissecting the Wnt pathway genetically (Rochard, Development, 2016; Kamel, Developmental Biology, 2013).
Advances in clinical treatment of congenital craniofacial malformations require improved understanding of the developmental genetic basis of facial morphogenesis. Our goal is to investigate fundamental genetic regulation of facial development, with focus on translating basic science discoveries to clinical treatments.
Vertebrate Craniofacial Morphogenesis
CLP Functional Genomics Pipeline
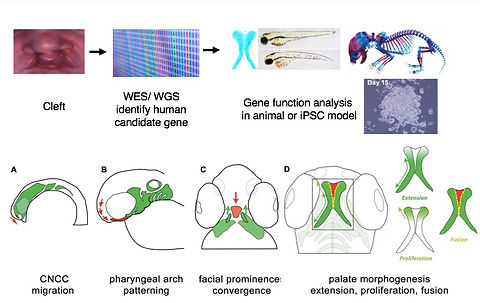
We are applying zebrafish to carry out high throughput functional genomics studies, to characterize human genes implicated in orofacial clefts (Mukherjee, Human Molecular Genetics, 2016; Gfrerer, Plastic and Reconstructive Surgery, 2014). We are also using zebrafish as the biological platform to identify chemicals that specifically regulate craniofacial morphogenesis, or even discover compounds that mitigate malformations (Kong, Chemistry & Biology, 2014).
With continued revolutionary advance in human gene sequencing approaches, there is pressing need to bridge the gap between whole genome analysis and clinical use of this data. Determination of pathogenicity of human gene variants is a major challenge in the field, limiting clinical usefulness of WGS information. We apply functional assays in iPSC and zebrafish models toward functional analysis of human gene variants associated with cleft and craniofacial anomalies (E BH Li, Plos Genetics, 2017).



